Ebola vaccines and pipelines: Where are we now?
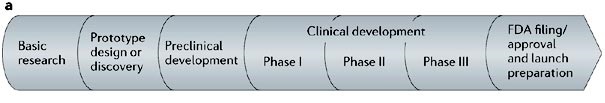
With the Ebola virus raging on in West Africa, scientists and clinicians around the world are racing to develop feasible treatments and vaccines. Here in the United States, there are currently two vaccines in Phase I clinical trials here at the National Institutes of Health. But what does that mean in terms of development? Are we close to deploying these therapies in Africa? To illustrate the typical path of development for drugs, here's a little graphic produced by Nature Drug Discovery:
As a side note, the NIH center where I work focuses on the stage just before Phase I, which is preclinical development. As the title implies, Phase I-III trials are conducted in human patients in the clinic. Briefly, here is a summary of the three different phases:
Phase I: Safety. In this phase, the vaccine is given to healthy individuals to determine if the vaccine is safe in humans and to establish what doses are safe. The virus in question is NOT given to the patient in this phase - this is simply to test if the vaccine by itself is safe.
Phase II: Efficacy. In this phase, the vaccine is given at full therapeutic dose to patients with the virus to determine whether or not it is effective. There are plans in motion to send doses of the vaccines to West Africa for this purpose.
Phase III: More efficacy! In this phase, the trial is expanded to a much larger amount of participants in a final determination of biological effectiveness.
These trials normally take years to complete, but due to the desperate need for therapeutics the "typical" pipeline is being greatly enhanced. The Phase 1 trials are underway, so hopefully GlaxoSmithKline and NewLink Genetics (the two companies with intellectual property rights in the vaccines) will be able to finish the entire pipeline as soon as possible. I'll go into more details as to how the current vaccines work in a few days, but keep an eye on the news to see if any new experimental drugs pop up!